Innovative biotechnology leader developing transformative new drugs
NYRADA INC. (NYR.ASX)
Nyrada Inc specialises in the discovery and development of small molecule drugs to address unmet medical needs in treatment areas with large commercial potential. In particular, Nyrada’s neuroprotection program aims to deliver the first treatment to prevent brain damage that can occur following a stroke, or from head trauma sustained in motor vehicle accidents, falls, and contact sports concussions. Nyrada also has ongoing studies in programs to lower cholesterol.
Current Drug Development Program
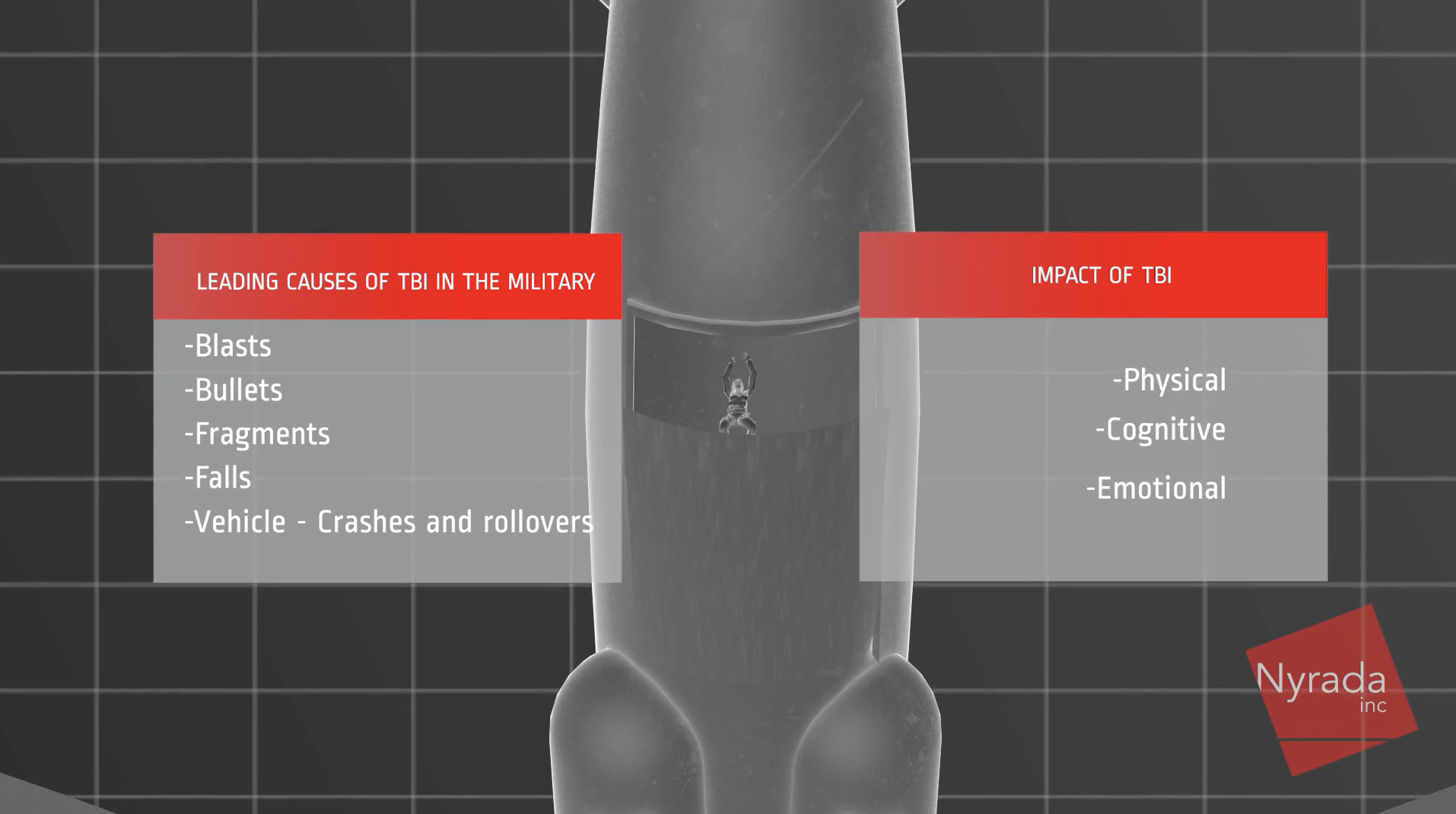
Neuroprotection: Development of the first-ever drug treatment to reduce brain damage following a stroke or after sustaining a traumatic brain injury (TBI) in a motor vehicle or other accident, during contact sports, or from bullet/shrapnel wounds and blast injuries sustained by military personnel during active service. The aim is to improve patient survivability, reduce the level of disability, and shorten recovery time.
Nyrada has completed a preclinical stroke study to assess the efficacy of NYR-B103.
The study showed a significant neuroprotective signal providing strong evidence of efficacy.
Latest Nyrada NewsKEY INVESTMENT INSIGHTS
Clear Business Strategy
Nyrada is focused on drug discovery developing innovative new treatments for which there is a large unmet clinical need and substantial market potential. The company aims to develop best-in-class drugs focussing on neuroprotection (reducing brain injury) and cardiovascular disease (lowering cholesterol).
Significant Investment Upside
There is potential for significant share price upside with the development of a first-in-class neuroprotectant drug with an ability to reduce the damage inflicted by excitotoxicity. Any deal is likely to include substantial up-front payments as well as significant ongoing royalties.
Scalable Business Model
Nyrada has an opportunity to offer a treatment for brain injury that prevents secondary brain damage and its devastating and life-long effects. With a market that currently accounts for US$100 billion in direct and indirect costs, the level of interest in the development of a treatment for brain injury will be very high across the entire medical industry. There is also likely to be a significant level of interest from professional and amateur sports organisations and the military, as demonstrated by the company’s collaboration with the Walter Reed Army Institute of Research (WRAIR) and the UNSW. WRAIR, the medical research arm of the US Dept of Defense reports that traumatic brain injuries are common in the military with 1 in 25 soldiers suffering a TBI during active service.
Successful Management
The Nyrada Board and management team include leading figures in biotech, both here in Australia and the US, who have a proven track record of success in commercialising innovative technology.
Nyrada’s CEO, James Bonnar, a chemist by training, brings 25 years of broad professional experience in the biotechnology industry. He has worked in preclinical drug development, product manufacturing, regulatory affairs, and clinical-stage drug research, most recently for Neuren overseeing the development of treatments for traumatic brain injury and neurodevelopmental disorders.
The Nyrada Board is a diverse mix of successful international entrepreneurs and dealmakers with proven track records in the pharmaceutical industry. Christopher Cox was the Chief Commercial Officer for The Medicines Company which developed the cholesterol-lowering drug Inclisiran®, a program that was acquired by Novartis in late 2019 for US$9.7B. At the time it was the largest ever acquisition deal for a company with a single drug in development. Chris has extensive industry contacts and this is an obvious benefit for Nyrada. Marcus Frampton is the Chief Financial Officer for the largest sovereign wealth fund in the US, The Alaska Permanent Fund, and has a proven record in identifying and backing successful biotech companies. Companies they have supported include Codiak BioSciences which is developing new medicines using exosomes and Juno Pharmaceuticals, an Australian company focused on the supply of off-patent drugs to hospitals. Dr. Rüdiger Weseloh works in commercial development for Merck KGA in Germany so he has a lot of experience negotiating and securing deals with pharmaceutical companies..
Nyrada’s Scientific Advisory Board comprises some of the world’s most respected scientific thought leaders from Australia, The USA, France, and Japan. The Chair of the board, Prof. Gary Housley, is the Chair in Physiology at the University of New South Wales Sydney (UNSW) and is a founding director of the Translational Neuroscience Facility and has over 30 years of experience. Prof. David Burke, currently a Professor of Neurology at RPA and the Sydney Medical School and was President of the Australian & New Zealand Association of Neurologists from 2005-2007. Prof. Gilles Lambert, Prof. Junichi Nabekura, and Dr. Jim Palmer bring expertise in cardiovascular, neuroscience, and drug discovery programs, respectively.
High Barriers To Entry
Nyrada has a strong intellectual property protection strategy with Patent Cooperation Treaty (PCT) and provisional patents filed for both its brain injury and cholesterol-lowering drugs. These are composition of matter patents that provide the highest level of protection and essentially mean that Nyrada owns the small molecule. A US patent for the cholesterol-lowering PCSK9i inhibitor has been granted. There is also a second patent which is in the provisional stage. In terms of the brain injury drug, the company has a provisional patent. The patents are recent and therefore provide up to 20 years of protection, which can be extended in certain circumstances. On February the 28th 2024, Nyrada announced positive results from its preclinical study evaluating the efficacy of its Brain Injury Program drug. Not only did the study support NYR-B103’s favourable safety profile (no drug related adverse effects) but also showed a significant neuroprotective signal providing strong evidence of efficacy.
Neuroprotection – Brain Injury Drug
Nyrada’s is developing a new and novel drug that reduces the long-term disability associated with stroke or traumatic brain injury (TBI) by limiting the number of brain cells that die post-injury. Currently, no treatments exist for TBI therefore the global market potential for a new drug that reduces the damage to the brain following a stroke or injury is very large.
Currently, approximately 15 million people suffer stroke worldwide each year. Of those, approximately one-third will die shortly after suffering a stroke, one-third will recover with little or no permanent disability, and one-third will be permanently disabled requiring long-term support and assistance. The neuroprotection drug development program aims to improve patient outcomes and increase the likelihood of recovery, shorten rehabilitation times and reduce the economic burden to the health system.
In the US, a stroke occurs every 40 seconds and a TBI every 15 seconds. The combined economic burden for stroke and TBI in the US amounts to more than US$100 billion in direct and indirect costs.
Concussion or mild TBI in contact sports accounts for 80% of all TBI cases and is a major cause of cognitive impairment and long-term physical illness. Repeated concussions lead to chronic traumatic encephalopathy (CTE) and brain degeneration caused by repeated head traumas. The incidence of concussion can range from 14.8-28.3 per 1000 player match hours. The economic burden of costs for rehabilitation and ongoing care for people who have suffered a TBI is very high.
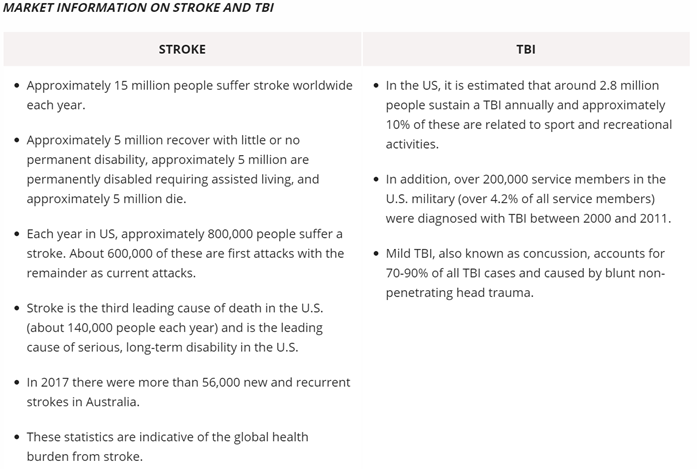
Nyrada hopes to reduce long-term disabilities that occur in people who have had a stroke or traumatic brain injury by limiting the extent of brain cell loss post-injury. The program aims to increase the chances of recovery by reducing secondary brain damage and rehabilitation times and the burden on healthcare systems as a result.
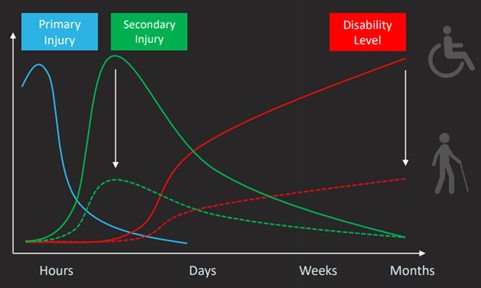
The drug would be taken over 3-4 days beginning a soon as possible after a brain injury to disrupt and reduce excitotoxicity, which is largely responsible for secondary brain damage to the brain. Excitotoxicity is a common occurrence after stroke or traumatic brain injury. Chemicals are released from dead and dying brain cells, which have been impacted by the original injury. The chemicals affect healthy brain cells that cover a larger area than the core injury causing the death of brain cells. Long-term disabilities result from an almost doubling in size of the original injury.
Secondary brain injury can more than double the total area of brain injury following a stroke or TBI and there is currently no treatment to stop cell death. Nyrada has developed a novel family of molecules, which can stop the excitotoxicity process.
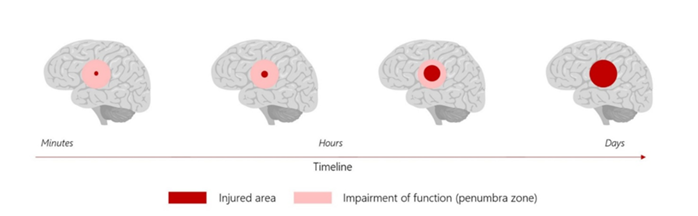
In a laboratory study, Nyrada’s drug candidates showed the ability to cross the blood-brain barrier and achieve concentrations anticipated to be therapeutic.
Collaboration Agreement with the Walter Reed Army Institute of Research & UNSW
A Collaboration Agreement with the specialist TBI research team at Walter Reed Army Institute of Research (WRAIR) & UNSW Sydney has been successfully negotiated. This will enable Nyrada to examine the ability of its lead neuroprotection compound to minimise the process responsible for secondary damage to the brain amongst military personnel who suffer a TBI during their active service careers. The agreement aligns Nyrada with WRAIR’s mission to develop ground-breaking treatments to mitigate the impacts of traumatic brain injury which occurs in 1 in 25 service members. The agreement may also provide Nyrada with access to non-dilutive funding to further develop its neuroprotection program.
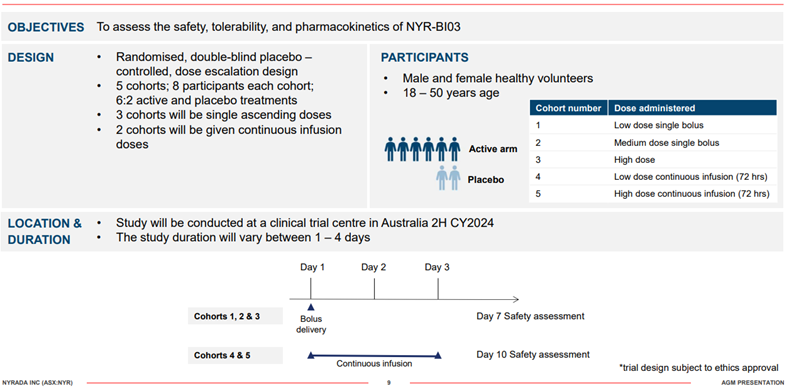
Brain Injury Preclinical study and Phase 1 Study Design – NYR-B103
In February 2024 Nyrada completed a preclinical stroke study to assess the efficacy of NYR-BI03. The study showed a significant neuroprotective signal providing strong evidence of efficacy. The study, conducted in collaboration with UNSW Sydney, involved inducing a focal ischemic stroke using a photothrombotic model (a minimally invasive and reproducible way of inducing focal brain injury in test animals). A total of 16 test animals were treated with either NYR-BI03 or a placebo 30 minutes following the induced brain injury, with treatment conducted for 72 hours via continuous intravenous infusion. Magnetic Resonance Imaging (MRI) was performed to quantify the brain injury in drug-treated and placebo animals. The MRI data determined tissue damage in the penumbra region, the area of secondary brain injury that the NYR-BI03 neuroprotection drug targets. The results showed that a statistically significant level of neuroprotection was achieved with NYR-BI03 rescuing an average of 42% of the brain injury in the penumbra region of the brain. All animals survived the brain injury and drug treatment with no drug-related adverse effects reported.
These analyses were performed blinded, where the experimenter did not know if a particular animal received NYR-BI03 or a placebo.
Subject to the successful completion of Good Laboratory Practice (GLP) safety testing, Nyrada will commence a Phase I human clinical trial for NYR-BI03. The Company has already undertaken necessary planning and engaged a Contract Research Organisation for this purpose. The target commencement of a Phase I trial is anticipated to start in the second half of the 2024 calendar year. The data from the GLP studies and Phase I trial will support the Phase II trials of NYR-BI03 for both TBI and stroke indications.
Drug Discovery
In addition to Nyrada’s lead Cardiovascular and Neuroprotection drug development programs, the Company is investigating drugs to treat pain associated with peripheral nerve damage (Inflammation/Pain) and autoimmune diseases such as psoriasis (Autoimmune).
The Inflammation/Pain and Autoimmune programs are part of a larger pipeline of potential drug candidates that Nyrada continues to evaluate and develop while focusing on the progression of its two lead drug development programs.
Further information concerning Nyrada’s drug development program can be obtained from following the links below.
KEY PEOPLE
-

John Moore
Non Executive Chairman
John has extensive industry expertise having been involved in multi-million dollar acquisitions. In 2002, Edson Moore Healthcare Ventures acquired sixteen interests in biotech corporations from Elan Pharmaceuticals, to which he was a co-founder for a sum of $148 million. John was CEO of Acorn Energy from 2006 to 2015, in which CoaLogix sold for $101 million after it was acquired for $11 million. He is currently active on 10 company boards and has been the head of 12 different companies in his career. He was educated at Rutgers State University in New Jersey.
-

James Bonnar
CEO
James has 25 years of experience in the global life sciences industry before joining Nyrada in February 2018. He has held various director-level roles across a range of functions, most recently at Neuren Pharmaceuticals where he worked for 11 years overseeing the development of a drug to treat traumatic brain injury and neurodevelopmental disorders. Before this, he held various senior roles at several pharmaceutical and healthcare product companies in New Zealand, China, and the UK. He brings an extensive scientific focus to the company and has experience in leading teams from discovery and early-stage development and through clinical development.
-

Christopher Cox
Non Executive Director
Chris has significant industry experience in mergers and acquisitions and corporate governance. He has received many industry awards such as one from The American Lawyer as one of the “Dealmakers of the Year” for his role in the Irish drug maker Elan Corporation sale to Perrigo Company. He was also named an M&A Atlas Top 50 Global M&A Lawyer for 2014.
He is currently CEO of Symphony Capital Holdings, LLC, a company involved in biotechnology, network security, and entertainment. Chris brings expertise in communication, corporate advisory, and restructurings with experience across foreign and domestic transactions.
-

Marcus Frampton
Non Executive Director
Marcus currently holds the position of Chief Investment Officer of the Alaska Permanent Fund Corporation. He has held roles at Lehman Brothers in an investment banking capacity, and private equity with PCG Capital Partners.
Marcus is an avid follower and investor in micro-caps with personal publications covering micro-caps with a private monthly periodical covering this sector in the market. He holds a Bachelor’s degree in Economics from the University of California, Los Angeles.
He currently is a shareholder of Scientific Industries, Inc., and holds a position as a director on the board.
-

Benny Evison
Chief Scientific Officer
Benny joined Noxopharm as a Director of Preclinical (Non-oncology) and subsequently joined NYR when it was formed. He has held a great passion for science from his early years and obtained a Bachelor of Medical Science with Honors as well as a Ph.D. from La Trobe University.
After a career move to Memphis, Tennessee in the USA, he developed a strong inkling to conduct and develop meaningful scientific research following on from his postdoctoral fellowship at St Jude Children’s Research Hospital. At the hospital, he worked to develop drugs specifically involving DNA repair to improve the activity of existing chemotherapies.
-

Dr Gisela Mautner
Non- Executive Director
Gisela is an international business leader with significant experience developing and launching new pharmaceutical products, and delivering successful corporate strategies in highly competitive global markets. She is currently Chief Executive Officer at Noxopharm Ltd (ASX:NOX) and has over thirty years’ leadership experience in business, along with medical and scientific research.
Dr. Mautner has held senior positions with Amgen, Bayer, Siemens Medical Solutions and Merck/MSD generating successful commercial and scientific outcomes. She is currently the Past-President of the Australian Pharmaceutical Physicians Association (APPA), a Fellow of the Australasian College of Physician Executives and a Member of the Australian Institute of Company Directors and the CEO Institute.
-

Dr. Rüdiger Weseloh
Non-Executive Director
Rüdiger has over 14 years of experience in the pharmaceutical industry, overseeing drug development and manufacturing across Oncology, Rheumatology, Neurodegenerative diseases, and Fertility. Ruediger in his time with Merck KGaA has been the lead on over 50 transactions for the pharmaceutical division. Before this he was a Biotech Pharma Equity Analyst, giving him a breadth of experience.
He has a diploma in Biochemistry from the University of Hannover and a Ph.D. in Molecular Neurobiology, obtained at the Center for Molecular Neurobiology in Hamburg.
-

Ian Dixon
Non Executive Director
Dr. Ian Dixon has a Ph.D. in biomedical engineering from Monash University, an MBA from Swinburne University, and professional engineering qualifications. Ian has over 20 years of experience as a biotechnology entrepreneur within Australia. As a co-founder of Nyrada, he has co-invented the technology that is behind the Cholesterol Lowering Program, giving him the most insightful understanding of PCSK9 inhibitors.
Ian has had great success in translating technology challenges into valuable businesses and respected intellectual property.